Bezisterim (NE3107)
Our lead asset, bezisterim (NE3107), is a novel, orally administered small molecule that is both anti-inflammatory and insulin-sensitizing, and is currently being studied in Alzheimer’s disease, Parkinson’s disease and Long COVID. As part of the clinical trials in these core indications, we’re also exploring bezisterim’s potential impact on longevity by measuring its ability to reduce DNA methylation in study participants.
In clinical trials, many patients treated with bezisterim experienced:
- Reduced inflammation and the associated insulin resistance
- Improved motor control and “morning on” symptoms in Parkinson’s disease (PD)
- Improved cognition and function, lowered amyloid β and p-tau levels, and improved brain imaging scans in Alzheimer’s disease (AD)
- Lowered DNA methylation levels
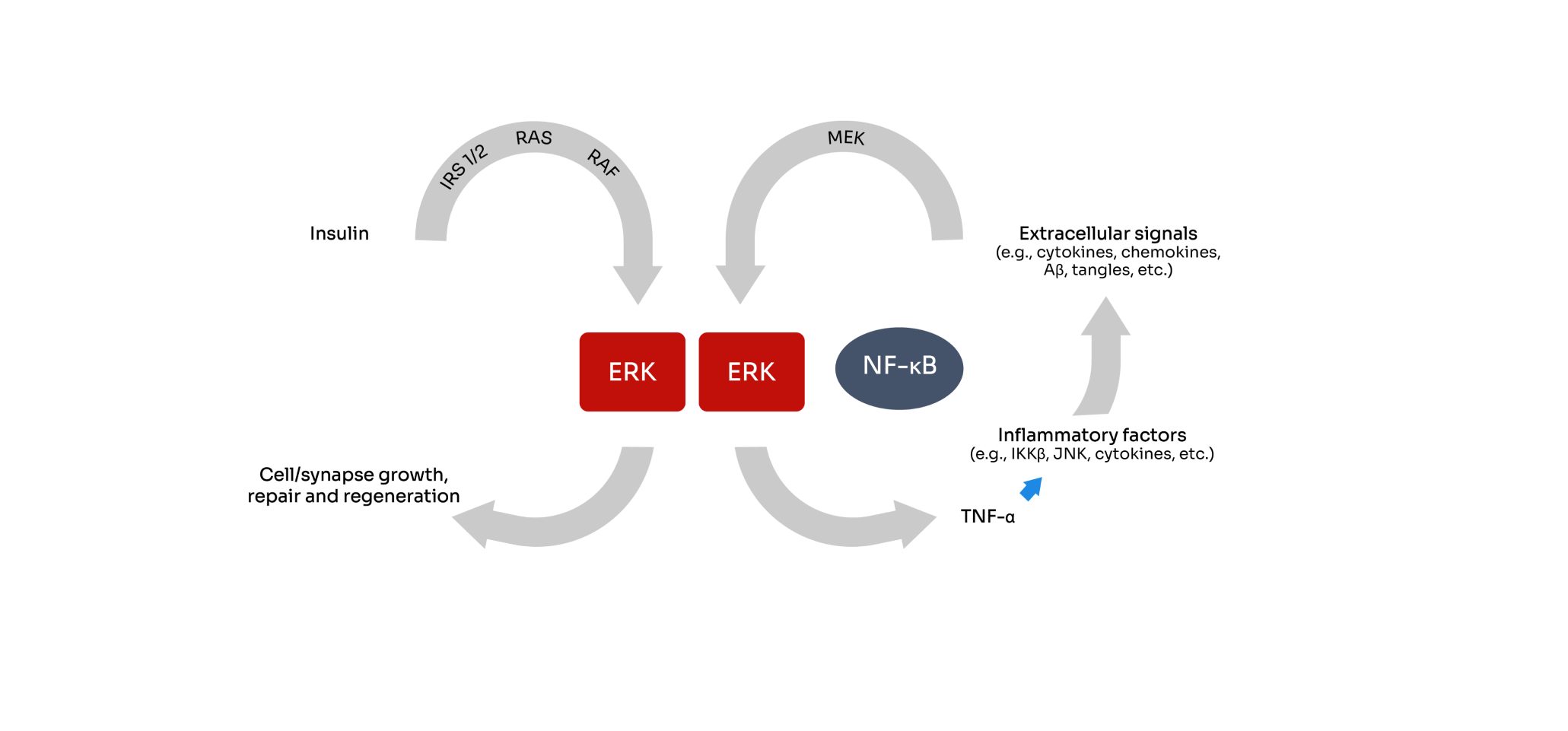
Bezisterim is an oral small molecule, blood-brain barrier (BBB) permeable, anti-inflammatory insulin sensitizer that binds extracellular signal-regulated kinase. Bezisterim has been shown to selectively inhibit inflammation-driven ERK- and NF-κB-stimulated inflammatory mediators, including tumor necrosis factor alpha (TNF-α), without inhibiting their homeostatic functions.
Rationale for TNF-α as a Therapeutic Target
An extensive body of evidence shows that both inflammation and insulin signaling are central to the pathophysiology or symptom development of an increasing number of conditions.
Far-reaching impact of TNFα-mediated chronic low-grade inflammation
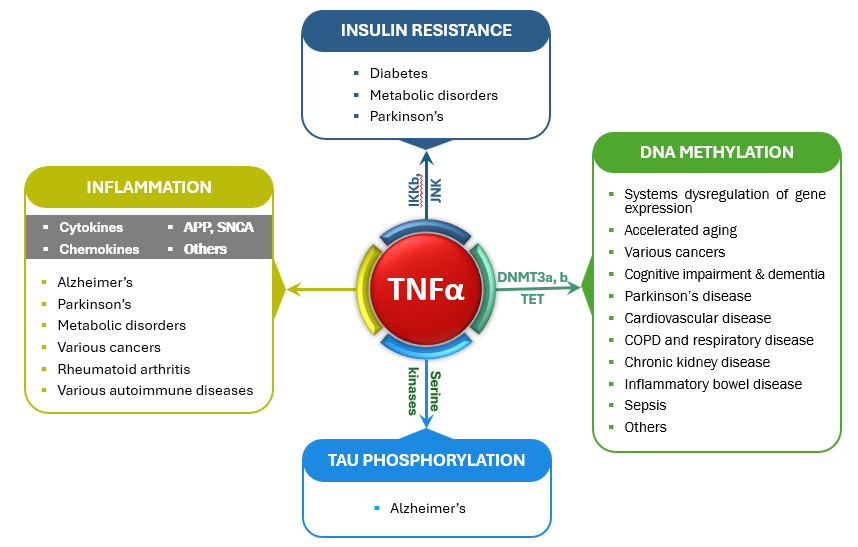
We believe TNFα-mediated chronic low-grade inflammation is the starting point for many things going wrong in the body. Chronic overproduction of TNFα has been shown to:
- Drive insulin resistance associated with diabetes and Parkinson’s
- Create a continuous inflammatory cycle by stimulation the production of various cytokines, chemokine, etc.
- Stimulate the kinases that turns Tau into phosphor-Tau in Alzheimer’s disease
- Activate the agents that add methyl group onto the surface of our DNA to drive DNA methylation, which leads to an accelerated aging process
Where We’re Targeting Inflammation and Insulin Signaling
- Both low dopamine in the brain and inflammation and the associated insulin resistance are required to create Parkinson’s symptoms. Since clinicians have never had a tool to reduce the insulin resistance, the only way to address Parkinson’s symptoms over the past 5 decades has been to increase the level of dopamine through the use of the drug levodopa.
- Bezisterim reduced inflammation and enhanced insulin resistance in animal models, and thus may offer a new treatment approach for Parkinson’s disease.
- Preclinical animal studies showed bezisterim was equally effective at restoring muscle control as levodopa, and that bezisterim in combination with levodopa accorded the greatest motor control. Furthermore, non-human primates treated with the combination saw less levodopa induced dyskinesia. Bezisterim also showed potential for a neuroprotective activity that promoted the survival of twice as many neurons in the substantia nigra versus placebo treatment.
- In a Phase 2 study, roughly one-third of patients younger than 70 years old treated with bezisterim and levodopa retained motor control upon waking up in the morning whereas none of the patients given just levodopa had morning control of their muscles (p=0.02) because levodopa wore off overnight as patients slept. Patients treated with the combination also experienced a ~5 point advantage on the motor score (a clinically significant and dramatic difference) on the Parkinson’s disease rating scale compared to patients treated with levodopa alone.
- Because both inflammation and insulin signaling are thought to contribute to dementia in Alzheimer’s disease, we believe that our clinical trials may show that bezisterim has the potential to slow the progression of Alzheimer’s disease, and that it may improve cognition and function related to activities of daily living.
- In a trial with 57 subjects who completed the trial per our protocol, patients treated with bezisterim for 6 months saw a 68% slowing of cognitive decline compared to placebo. To provide some context, the patients treated with FDA-approved drug Leqembi for 18 months saw a 27% slowing of cognitive decline in their clinical trial.
- The most commonly observed adverse event has been a transient, mild headache reported 10% of bezisterim-treated patients.
- Chronic inflammation is one of the main hypotheses that researchers have proposed to explain the persistence of symptoms in Long COVID. The expression of proteins associated with inflammation (LGALS9, CCL21, CCL22, TNF, CXCL10 and CD48) and immune regulation (IL1RN and CD22) have been shown to be elevated in individuals with Long COVID versus full-recovered individuals.
- Specifically in individuals with “brain fog,” sustained systemic inflammation and persistent localized blood-brain-barrier (BBB) dysfunction are key physiological features. Thus, drugs modulating inflammation, and that work to regulate the BBB integrity, could represent potential therapeutic mechanisms for treating neurological symptoms of Long COVID.
- Age deceleration is the difference between a person’s biological age as measured by various DNA methylation clocks and their actual age since birth. A lower value is preferable and suggests that the biological age is lower than the actual age.
- Our data show that patients treated with bezisterim experienced an age deceleration advantage over patients treated with placebo on five biological clocks, including -3.68 years advantage on the SkinBlood Clock (p=0.017), -4.77 years advantage on the Inflammation Age Clock 4 (p=0.022), -5.0 years advantage on the Hannum Age Clock 5 (p=0.006), -1.92 years advantage on the GrimAge Clock 6 (p=0.068), and -3.71 years on the PhenoAge Clock 7 (p=0.081).

